Pharmaceutical Contamination
Contamination in pharmaceutical products can lead to significant risks,...
Explore the Pharmaceutical RCA Blog, your trusted resource for Root Cause Analysis (RCA) in the pharmaceutical sector. Gain access to a comprehensive library of RCA templates tailored to pharma-specific challenges, including deviations, batch failures, equipment breakdowns, and compliance issues. Learn proven methodologies for investigating incidents, ensuring GMP compliance, and optimizing manufacturing and quality control processes. Use our intuitive fishbone diagrams to navigate safety, regulatory, and operational hurdles with clarity. Elevate your quality assurance and operational excellence with ProSolvr, a Generative AI-powered visual RCA platform. Discover hidden causes, drive continuous improvement, and ensure product integrity with us as your RCA partner.
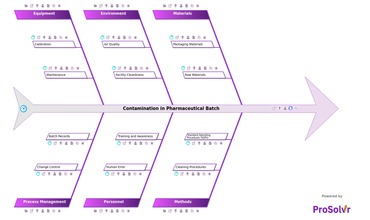
Contamination in pharmaceutical products can lead to significant risks,...
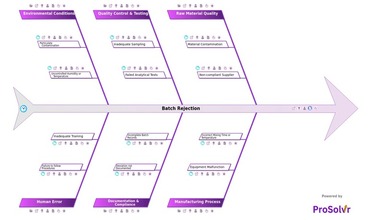
In the pharmaceutical industry, batch rejection, also referred...
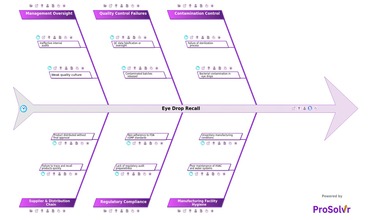
In May 2022, the CDC began tracking an unusual outbreak...
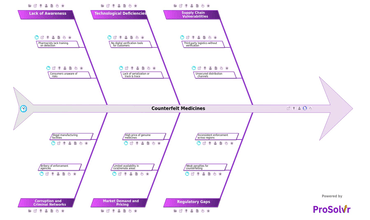
Counterfeit medicines remain one of the most pressing threats...
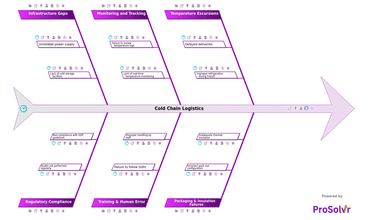
Cold chain logistics refers to the transportation and storage...
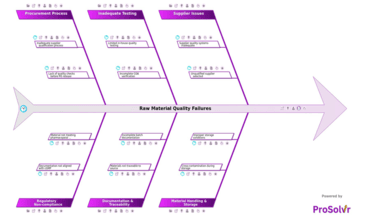
Raw material quality failures in the pharmaceutical industry often...
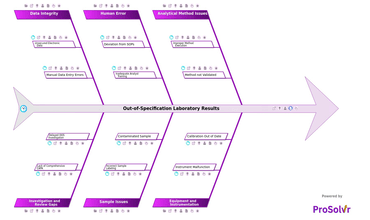
Out-of-Specification (OOS) laboratory results occur when test outcome...
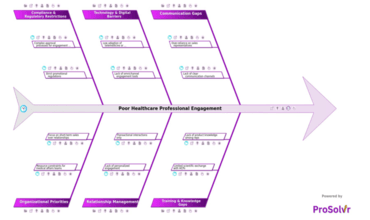
Poor healthcare professional (HCP) engagement is one of the most...
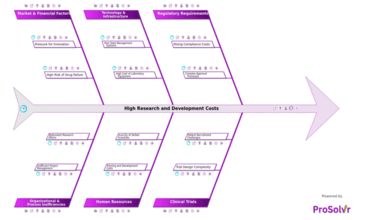
High research and development (R&D) costs are one of the most critical...
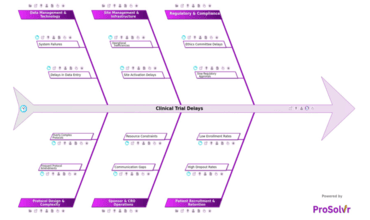
Clinical trial delays remain a significant challenge in the...
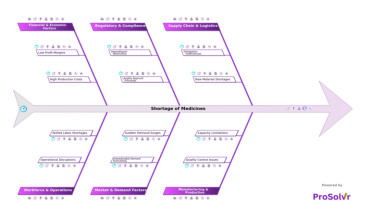
Shortage of medicines is a critical challenge in the pharmaceutical...
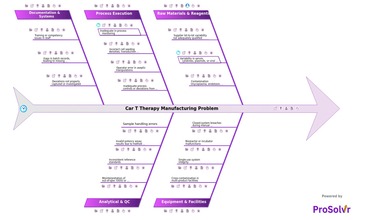
CAR T therapy manufacturing involves complex, multi-step processes...