RCA of Cold Chain Logistics
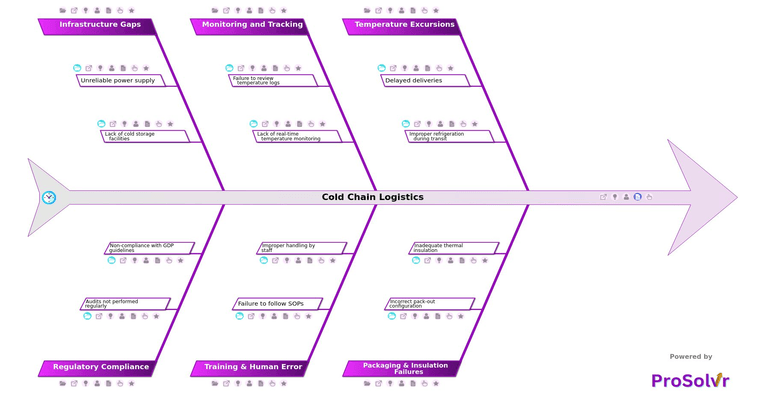
Cold chain logistics refers to the transportation and storage of temperature-sensitive pharmaceutical products under strictly controlled conditions, typically between 2°C and 8°C. Maintaining this cold chain is vital to preserve the efficacy, stability, and safety of critical medicines such as vaccines, biologics, and insulin. Any temperature excursion—whether due to delayed deliveries, improper refrigeration during transit, or cooling equipment malfunction—can compromise product quality and pose risks to patient safety. These incidents often stem from traffic or customs clearance issues, packaging and insulation failures, or deviations from validated SOPs.
The consequences of cold chain failures can be far-reaching, including product recalls, regulatory penalties, reputational damage, and financial loss. For example, an incorrect pack-out configuration or the use of substandard packaging material can lead to inadequate thermal insulation, exposing products to unsafe conditions. Compounding this, many organizations struggle with monitoring and tracking, where temperature logs are only reviewed post-delivery, or lack real-time GPS or IoT-based tracking. These issues are often intensified by human error, such as improper handling by staff or failure to follow SOPs, often due to insufficient training and lack of ongoing audits.
A GEN-AI powered root cause analysis platform like ProSolvr can help organizations trace and resolve such failures systematically. Using tools like the Fishbone (Ishikawa) diagram and Six Sigma methodologies, ProSolvr enables teams to examine categories including training gaps, infrastructure breakdowns, and regulatory non-compliance. The investigation may uncover deeper issues like unreliable power supply, lack of cold storage facilities, or missing cold chain validation documentation. ProSolvr’s structured approach helps develop targeted Corrective and Preventive Actions (CAPA) while improving visibility and accountability across the cold chain.
By identifying and documenting root causes—whether it's non-compliance with GDP guidelines, absence of risk-based audit programs, or simply failure to maintain SOP discipline—ProSolvr empowers pharmaceutical and logistics teams to not just fix failures but to build resilience into their cold chain systems. In highly regulated, high-stakes environments, such post-incident learning is not optional, it is essential.
Who can learn from the Cold Chain Logistics template?
- Quality Assurance (QA) and Quality Control (QC) Teams: These professionals can use the RCA to understand how systemic breakdowns in temperature-sensitive logistics affect product integrity, and how structured analysis supports CAPA implementation to maintain compliance and prevent repeat failures.
- Supply Chain and Logistics Managers: They can gain insights into the importance of end-to-end visibility, contingency planning, and procedural adherence in ensuring the safe and timely delivery of pharmaceutical products under cold chain conditions.
- Regulatory Affairs Professionals: RCA helps them understand the downstream impact of regulatory non-compliance and the value of proactive auditing and documentation to meet global Good Distribution Practice (GDP) standards.
- Training and Compliance Officers: These individuals can use the analysis to develop targeted training modules and periodic audit checklists that address common operational pitfalls, helping staff remain vigilant and compliant in cold chain handling.
- Pharmaceutical Manufacturing Executives: RCA findings enable senior decision-makers to allocate resources, invest in infrastructure, and support risk-mitigation strategies that improve operational resilience and safeguard product quality.
- Technology and System Integration Teams: These teams can learn how digital tools like ProSolvr and AI-powered root cause analysis applications can be leveraged to visualize complex problems, automate CAPA processes, and foster continuous improvement in temperature-controlled logistics.
Why use this template?
Applications like ProSolvr elevate root cause analysis by offering an intuitive, AI-assisted platform to build and analyze Fishbone (Ishikawa) diagrams with speed and precision. Teams can collaborate visually, identify systemic and contributing causes, and drive effective CAPA planning. This ensures that every corrective, preventive, and investigative action is aligned with both regulatory expectations and business objectives.
Use ProSolvr by smartQED to effectively uncover, manage, and ultimately eliminate recurring cold chain failures in your organization—transforming incidents into lasting operational improvements.