RCA of Counterfeit Medicines
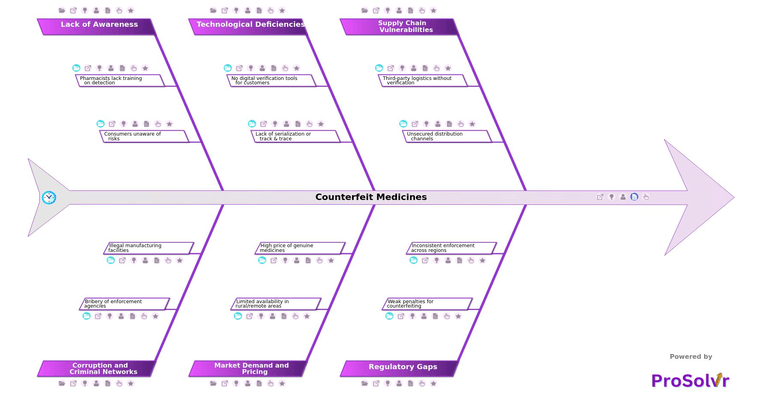
Counterfeit medicines remain one of the most pressing threats to global public health, especially in regions with limited oversight. These fake or substandard drugs, often containing incorrect or no active ingredients, can lead to treatment failure, increased antimicrobial resistance, or even death. Criminal networks exploit supply chain vulnerabilities. These include third-party logistics without verification, no background checks on vendors, and unsecured distribution channels lacking authentication mechanisms. The result is a dangerous ecosystem where counterfeiters thrive while patients are left exposed.
The rise of counterfeit medicines is fueled by a mix of regulatory gaps, technological deficiencies, and market pressures. Many regions suffer from weak penalties for counterfeiting and inconsistent enforcement across borders, leaving no deterrent for repeat offenders. On the technology front, the absence of digital verification tools, QR codes, serialization, and track and trace systems, combined with manual packaging, makes products highly vulnerable. Meanwhile, limited availability in rural areas and the high price of genuine medicines create demand that counterfeiters readily exploit. This is made worse by a lack of awareness, where pharmacists lack training on detection and consumers are unaware of the risks, due to insufficient public education.
When a counterfeit medicine incident is discovered, it becomes critical for pharmaceutical companies and supply chain stakeholders to conduct a structured, in-depth investigation. This is where ProSolvr brings clarity and control. As an AI-powered root cause analysis platform, ProSolvr enables teams to visually map the complex web of contributing factors, including illegal manufacturing, corruption, bribery of enforcement agencies, and gaps in distribution and education. Using frameworks like the Fishbone diagram and Six Sigma methodology, ProSolvr helps organizations identify true root causes, assign responsibilities, and track Corrective and Preventive Actions (CAPA), turning every counterfeit incident into an opportunity for systemic improvement, operational resilience, and future risk reduction.
Who can learn from the Counterfeit Medicines template?
- Pharmaceutical Quality Assurance Teams: To understand how to conduct structured post-incident investigations and implement effective Corrective, Preventive, and Investigative Actions (CAPA) to avoid future quality lapses.
- Regulatory and Compliance Officers: To learn how to identify systemic gaps in oversight, policy enforcement, and inter-agency coordination using structured root cause frameworks like fishbone diagrams.
- Supply Chain and Distribution Managers: To grasp how vulnerabilities in procurement, vendor management, and logistics can be systematically analyzed and mitigated post-incident.
- Healthcare Administrators and Hospital Pharmacists: To gain awareness of risks in medicine sourcing and handling, and how structured RCA can improve safety protocols and safeguard patients.
- Forensic Auditors and Investigative Journalists: To explore how deep-dive analysis methods like RCA can be used to trace accountability and identify weak links in regulatory and commercial ecosystems.
- Public Health Policy Makers and NGOs: To use the RCA template as a learning tool for designing awareness campaigns, policy reforms, and training programs aimed at preventing counterfeit drug circulation.
Why use this template?
The Counterfeit Medicines RCA template provides both a granular and holistic view of incidents, enabling teams to move beyond symptoms and identify true root causes. In a field where every error can have life-threatening consequences, structured and AI-powered RCA tools like ProSolvr are essential—not just for insight, but for systemic improvement.
ProSolvr transforms root cause investigations with speed, accuracy, and collaboration. By leveraging Gen-AI powered analysis, organizations can uncover failure patterns faster, prioritize actions intelligently, and eliminate recurring problems with confidence.
Use ProSolvr by smartQED to investigate critical issues, document findings, and implement CAPA that drives long-term change.