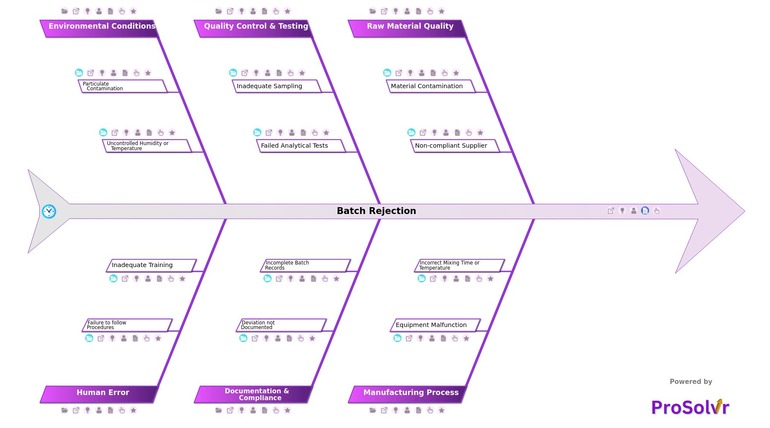
RCA of Batch Rejection
In the pharmaceutical industry, batch rejection, also referred to as pharmaceutical batch failure, occurs when a manufactured drug product fails to meet predefined standards of quality, safety, or regulatory compliance. These failures may arise due to raw material issues, manufacturing deviations, documentation errors, environmental conditions, or human mistakes.
While batch rejection serves as a safeguard against the release of substandard or unsafe products, the consequences are often costly. Pharmaceutical companies face delayed product availability, financial losses, increased regulatory scrutiny, and reputational damage. In highly regulated environments, repeated or unexplained batch failures can lead to GMP audits, warning letters, or even license suspensions.
Underlying causes such as material contamination, equipment malfunction, or deviation from manufacturing procedures are often not immediately obvious. This makes it critical for pharmaceutical teams to move beyond superficial fixes and conduct a structured investigation using Root Cause Analysis to identify the true contributing factors.
Today, leading quality assurance and manufacturing teams in pharma are turning to AI-powered root cause analysis platforms to accelerate investigations. Tools guided by fishbone diagrams and Six Sigma methodologies help teams systematically map contributing factors across key domains such as materials, methods, machines, environment, measurement, and human behavior.
This structured approach supports the creation of robust Corrective, Preventive, and Investigative Actions (CAPA), not only solving the current issue but also building resilience against future batch failures. An effective CAPA framework is essential for regulatory compliance, continuous improvement, and audit readiness.
This is where ProSolvr offers a powerful advantage. Purpose-built for root cause analysis in pharmaceutical and other regulated industries, the Gen AI powered ProSolvr platform enables teams to visually map root causes, uncover hidden dependencies, and collaborate across functions with ease. Unlike traditional tools, ProSolvr delivers clarity and structure to complex investigations while supporting digital CAPA planning aligned with GMP and quality standards.
With its AI-enhanced fishbone diagrams and intelligent RCA workflow, ProSolvr transforms problem-solving in pharmaceutical manufacturing. It helps ensure that batch rejection causes are not only identified but fully addressed through data-driven actions. The result is a smarter, more confident approach to pharmaceutical quality assurance that aligns with business goals, regulatory expectations, and patient safety requirements.
Who can learn from the Batch Rejection template?
- Quality Assurance (QA) Teams: QA professionals can gain insights into recurring compliance gaps, procedural weaknesses, and quality deviations, helping them improve oversight and reinforce good manufacturing practices.
- Manufacturing and Operations Teams: These teams can understand how variations in process execution affect product outcomes, enabling them to implement better control measures and refine standard operating procedures.
- Regulatory and Compliance Officers: RCA outcomes help this group ensure that documented corrective and preventive actions meet regulatory expectations and reduce the risk of non-compliance findings during audits.
- Training and HR Departments: By reviewing RCAs, training teams can identify knowledge or skill gaps and design focused learning modules that strengthen staff competency and procedural adherence.
- Procurement and Supplier Management Teams: These stakeholders can use RCA findings to assess and manage supplier performance, ensuring that materials sourced meet stringent quality and reliability standards.
- Research and Development (R&D) Teams: R&D professionals can use insights from RCAs to improve formulation robustness, process scalability, and design processes that are more resilient to real-world production variables.
Why use this template?
A comprehensive RCA with an application like ProSolvr ensures that CAPA is not merely reactive but strategically preventive. By identifying the root causes pharmaceutical companies can implement targeted training programs, reinforce procedural compliance, or invest in infrastructure upgrades. GEN-AI assisted applications ensure that these actions are not guesswork but data-informed and logic-driven. As a result, future batch rejections can be significantly reduced, ensuring product quality, regulatory compliance, and patient safety.
Use ProSolvr by smartQED to successfully navigate and resolve problems in your organization.