RCA of Clinical Trial Delays
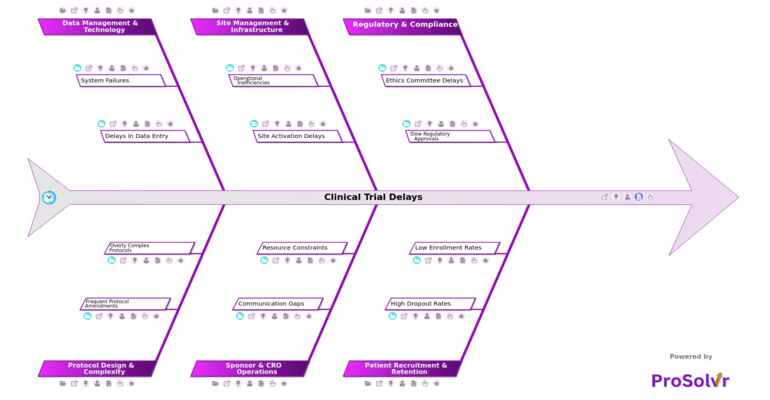
Clinical trial delays remain a significant challenge in the pharmaceutical industry, driving up costs, extending development timelines, and delaying patient access to vital therapies. These delays often stem from regulatory & compliance issues such as ethics committee delays, multiple review cycles, incomplete documentation, slow regulatory approvals, regional differences in regulations, and complex submission requirements. Each of these obstacles not only slows trial progress but also places financial and reputational strain on sponsors, investors, and stakeholders.
Recruitment and retention difficulties add further setbacks, with low enrollment rates, high dropout rates, and lack of patient awareness restricting participation. Burdensome visit schedules, limited patient engagement, and stringent eligibility criteria further reduce recruitment effectiveness. On the operations side, site management & infrastructure challenges including site activation delays, contract negotiation delays, lack of trained staff, inadequate monitoring, poor site coordination, and operational inefficiencies frequently derail trial initiation and execution.
Sponsor & CRO operations and data management processes introduce additional risks. Communication gaps, ineffective escalation processes, poor CRO-sponsor alignment, delayed decision-making, resource constraints, and insufficient staff allocation all contribute to trial delays. At the same time, data management & technology issues such as EDC downtime, inadequate IT support, delays in data entry, lack of system integration, and manual entry processes create further inefficiencies. Protocol design & complexity compounds the problem through frequent protocol amendments, inadequate feasibility assessments, changing regulatory requirements, overly complex protocols, difficult procedures for patients, and too many endpoints.
A GEN-AI powered root cause analysis (RCA), structured through a fishbone diagram and aligned with Six Sigma principles, can be invaluable in addressing these challenges. ProSolvr enables teams to systematically analyze the causes of clinical trial delays across regulatory, patient recruitment, site management, CRO operations, data management, and protocol complexity. ProSolvr empowers stakeholders to uncover underlying issues, align on effective Corrective and Preventive Actions (CAPA), and ensure that similar delays are avoided in the future. This structured approach helps organizations improve trial efficiency, strengthen collaboration, and accelerate time-to-market.
Who can learn from the Clinical Trial Delays template?
- Clinical Operations Teams: They can understand how inefficiencies in site activation, monitoring, and protocol management contribute to delays, helping them streamline trial execution.
- Regulatory and Compliance Officers: By reviewing causes like ethics committee delays and complex submission requirements, they can identify opportunities to strengthen documentation and approval processes.
- Patient Recruitment Specialists: They gain insights into challenges such as low enrollment rates and retention barriers, enabling them to design better engagement and outreach strategies.
- Data Management Teams: They can learn how EDC downtime, manual processes, and inadequate monitoring affect trial timelines, guiding improvements in data handling systems.
- CROs and Sponsor Relationship Managers: By analyzing issues like poor CRO-sponsor alignment and delayed decision-making, they can enhance collaboration and contract management practices.
- Project Managers: They can use the RCA findings to anticipate risks across multiple functions, ensuring better contingency planning and timely implementation of CAPA.
Why use this template?
The visual clarity of ProSolvr simplifies collaboration across teams and ensures that lessons learned from one delay can be systematically applied to future clinical trials, strengthening resilience and efficiency in pharmaceutical operations. By understanding the root causes of bottlenecks that create delays, organizations can save valuable time and resources while improving trial outcomes.
When medicines successfully pass clinical trials, researchers and pharmaceutical companies move one step closer to curing illnesses and delivering life-saving therapies to patients. GEN-AI powered applications like ProSolvr can play a pivotal role in helping pharmaceutical companies tackle clinical trial delays with structured root cause analysis for lasting improvements.
Use ProSolvr by smartQED to overcome bottlenecks, accelerate trial efficiency, and build a healthier tomorrow.