RCA of Label Mix-Up in Pharmaceutical Industry
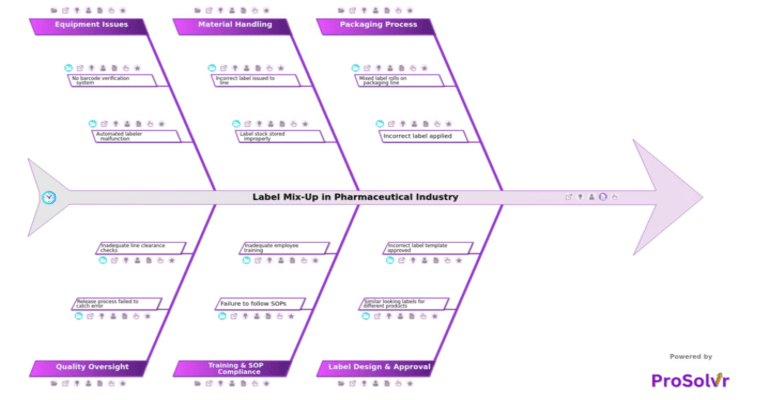
Label mix-ups in pharmaceuticals are serious failures that can result in the wrong medication or dosage reaching patients, putting lives at risk and exposing companies to regulatory penalties, recalls, and long-term reputational damage. Despite tight controls, these incidents still occur—and when they do, they demand more than surface-level fixes. Root causes often span multiple domains and involve a combination of human error, procedural lapses, and systemic breakdowns across manufacturing, packaging, and quality functions.
Such incidents are rarely the result of a single fault. They often involve packaging errors like incorrect label application or operators selecting the wrong label roll. Label design flaws—such as similar-looking layouts, lack of color differentiation, or poor template control—can make it easy to confuse products. Material handling missteps, including improper segregation of labels, multiple label types in one area, or incorrect label issuance to the line, contribute further. Training gaps and non-compliance with SOPs, along with equipment issues like labeler malfunctions or the lack of barcode verification, can all play a role. Compounding this are quality oversight misses, such as skipped inspections or failed reconciliation of label counts.
Root Cause Analysis (RCA) is critical to uncovering these contributing factors in a structured, repeatable way. Rather than relying on ad-hoc discussions or unstructured documentation, a disciplined RCA approach allows teams to systematically explore all potential failure points—across people, process, materials, methods, equipment, and environment. Visual tools like fishbone diagrams are especially effective in this regard, as they enable teams to map interconnected causes clearly and facilitate more productive, cross-functional collaboration.
This is where ProSolvr adds value. As an AI-assisted visual RCA platform, ProSolvr helps teams structure their analysis, organize brainstorming outcomes, and document causes and CAPA plans within a clear, categorized framework. It guides users in building fishbone diagrams aligned with Six Sigma principles and supports consistent RCA practices across investigations. While the root cause insights still come from human expertise, ProSolvr eliminates the friction of manual documentation, reduces ambiguity, and ensures that every investigation is well-organized, audit-ready, and easier to act upon. In critical scenarios like label mix-ups, that clarity makes all the difference.
Who can learn from the Label Mix-Up in Pharmaceutical Industry template?
- Quality Assurance Teams:They can gain insights into how lapses in compliance and procedural checks can lead to critical errors, reinforcing the importance of thorough documentation, auditing, and line clearance protocols.
- Production and Packaging Personnel: This group can understand how operational discipline, adherence to SOPs, and careful attention during labeling and packing can prevent downstream risks and product recalls.
- Regulatory and Compliance Officers: They can use the RCA to assess how internal controls align with regulatory expectations and identify areas where preventive systems must be strengthened to avoid non-compliance
- Training and HR Departments: RCA findings help highlight training gaps and competency issues, guiding the development of more effective onboarding, periodic refresher sessions, and employee skill assessments.
- Engineering and Equipment Maintenance Teams: They can identify how equipment reliability and system design influence labeling accuracy, motivating the adoption of more robust verification systems and better maintenance schedules.
- Management and Decision-Makers: Leaders can use the RCA to evaluate organizational culture, resource allocation, and the effectiveness of cross-functional collaboration, enabling more strategic investments in quality systems and continuous improvement.
Why use this template?
Label mix-ups aren’t just procedural errors as they represent serious patient safety risks. Addressing them effectively requires a structured, cross-functional approach to problem-solving. The Label Mix-Up RCA template, built around proven tools like fishbone diagrams and supported by platforms like ProSolvr, brings consistency, speed, and clarity to what is otherwise a complex investigation.
By aligning your RCA with Six Sigma principles and organizing your findings in a visual, collaborative format, ProSolvr helps teams not only resolve the current incident but also build stronger preventive practices. It standardizes the investigative process, encourages thorough root cause identification, and simplifies the documentation of CAPA outcomes.
With ProSolvr by smartQED, pharmaceutical teams can drive more effective resolution and ultimately, safer products.