RCA of Out of Specification Laboratory Results
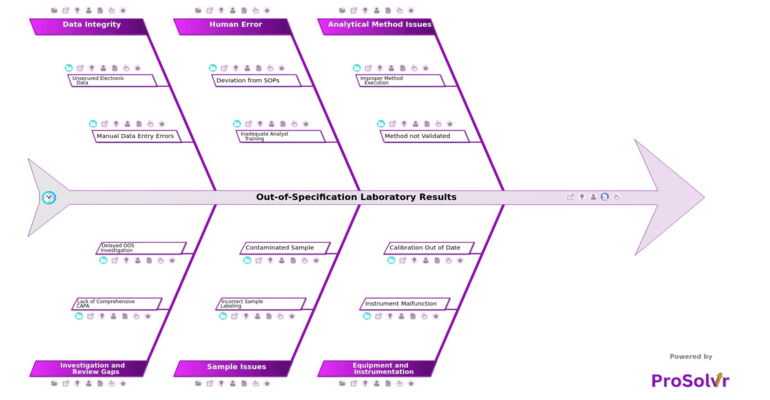
Out-of-Specification (OOS) laboratory results occur when test outcomes fall outside predefined acceptance criteria—whether set by regulatory agencies or internal quality systems. In pharmaceutical manufacturing and quality control labs, these deviations are more than just compliance risks. They often reveal deeper failures in process design, method execution, or laboratory discipline. A common contributor is improper method execution, such as incorrect sample preparation or failure to follow procedural steps. In other cases, the root lies in unvalidated methods with unoptimized parameters, factors that can introduce inconsistencies and obscure the true quality of the product.
When an OOS result emerges, the root cause is rarely obvious. It may be concealed by multiple layers of operational complexity. Equipment issues like instrument malfunction, often due to faulty sensors or detectors, can distort analytical readings. These issues are compounded when calibration protocols are missed or poorly followed, causing deviations that go undetected until too late. Human factors also play a critical role. Errors such as deviation from SOPs, lack of second-person verification, or insufficient analyst training often introduce variability. Even when processes are followed, issues like sample contamination or labeling mix-ups can trigger false OOS results and mislead investigations.
To truly resolve OOS events, teams need more than documentation checklists or anecdotal insights - they need a structured Root Cause Analysis (RCA) framework. This is where ProSolvr makes a difference. It supports quality and lab teams in analyzing issues after they occur by enabling visual mapping of root causes using an AI-powered fishbone (Ishikawa) diagram. By organizing potential causes across key categories such as analytical method, equipment, human error, sample integrity, data management, and investigation gaps, ProSolvr helps teams explore complex, interrelated failure points. This structure reduces the risk of premature conclusions and promotes systemic understanding.
Beyond structure, ProSolvr adds speed, transparency, and traceability to the investigation process. It helps teams track and resolve issues such as manual data entry errors, missing audit trails, or non-systemic CAPAs that often hinder OOS investigations. Instead of siloed documentation or disconnected action items, ProSolvr provides a shared workspace for collaborative RCA and resolution planning. The result is faster turnaround, fewer repeat deviations, and stronger compliance posture. For pharmaceutical teams navigating critical quality challenges, ProSolvr turns RCA from a reactive task into a proactive advantage.
Who can learn from the Out of Specification Laboratory Results template?
- Quality Assurance (QA) Teams: QA professionals can use insights from the RCA to identify systemic gaps in compliance, improve documentation practices, and ensure that corrective and preventive actions are robust and sustainable.
- Quality Control (QC) Laboratory Staff: Lab analysts and supervisors benefit from understanding the root causes behind testing failures, which helps them improve testing accuracy and minimize human or technical errors.
- Regulatory Affairs Personnel: This group can ensure that all investigations are thorough and CAPA plans are well-aligned with regulatory expectations. RCA findings help them strengthen compliance strategies and prepare for inspections or audits.
- Manufacturing and Production Teams: By reviewing OOS causes, manufacturing teams can make upstream improvements to prevent issues that could affect laboratory test outcomes.
- Training and Human Resources (HR) Departments: When RCA uncovers issues, it provides a clear signal for HR and training departments to enhance competency-based training modules and ensure employees are well-prepared for complex procedures.
- IT and Data Integrity Specialists: The findings can guide IT and compliance teams in strengthening digital systems, introducing proper audit trails, and reducing the risk of data-related non-conformances.
Why use this template?
ProSolvr brings visual clarity and structured collaboration to the RCA process, helping cross-functional teams build a shared, evidence-based understanding of OOS events. The platform enables seamless documentation of every step, making the investigation process traceable, audit-ready, and regulatory-compliant.
With embedded Generative AI, ProSolvr also assists in uncovering potential linkages between factors that may otherwise be overlooked. This intelligent guidance improves the accuracy and depth of root cause findings, allowing teams to develop stronger, more targeted CAPA strategies.
Use ProSolvr by smartQED to resolve Out-of-Specification issues effectively for safer pharmaceutical operations, better compliance, and ultimately, enhanced patient outcomes.