RCA of Car-T Therapy Drug Manufacturing Problem
CAR T therapy drug manufacturing involves complex, multi-step processes where even minor deviations can compromise product quality, patient safety, and treatment efficacy. Failures in raw materials, process execution, equipment, or quality control can disrupt production, leading to delays, batch failures, or regulatory non-compliance. Such issues directly affect treatment availability for patients with critical conditions and expose organizations to financial and reputational risks.
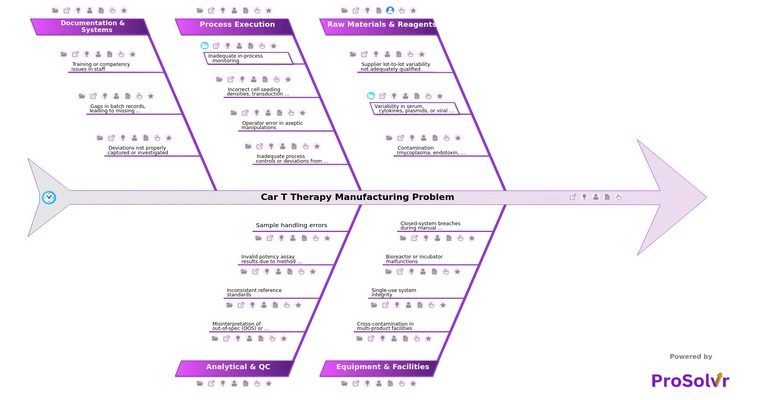
Root causes of CAR T manufacturing challenges span across raw materials, equipment, process execution, analytical testing, and documentation systems. For example, supplier lot-to-lot variability in cytokines or viral vectors can alter cell performance. Equipment malfunctions such as bioreactor failures or breaches in single-use systems may lead to contamination. Insufficient in-process monitoring of transduction efficiency or cell viability can compromise product potency. Similarly, operator errors during aseptic manipulations, gaps in documentation, and training deficiencies undermine reliability and traceability.
Regulatory and compliance hurdles also contribute, with import/export restrictions, sudden policy changes, country-specific trade barriers, lengthy approval cycles, and complex documentation requirements delaying market availability. On the market side, unpredictable demand forecasting, lack of real-time data, panic buying, and demand surges during epidemics or pandemics often overwhelm supply systems. Financial and economic pressures, such as price controls, low profit margins, high production costs, and raw material price fluctuations, add further strain. Finally, workforce and operational challenges including absenteeism during pandemics, labor strikes, high staff turnover, and shortage of trained technicians amplify disruption risks.
A structured RCA, supported by ProSolvr’s GEN-AI engine, helps systematically uncover and categorize these causes. By visualizing interdependencies and aligning with CAPA best practices, organizations can address systemic weaknesses, ensure product quality, and maintain compliance while accelerating delivery of these life-saving therapies.
Who can learn from the Car T Therapy Manufacturing Problem template?
- Manufacturing Teams: Gain insights into equipment, process, and monitoring challenges.
- Quality Control & Assurance: Improve detection of assay variability and OOS/OOT data handling.
- Regulatory Affairs: Strengthen compliance by ensuring traceable, gap-free documentation.
- Training & HR Teams: Identify operator competency gaps and design effective training.
- Supply Chain & Procurement: Ensure robust supplier qualification and multi-source strategies.
Why use this template?
- By combining structured RCA with ProSolvr’s GEN-AI powered insights, CAR T therapy manufacturers can go beyond surface-level issue fixing. This approach ensures:
- Early detection of variability or contamination.
- Systematic prevention of equipment and operator-driven errors.
- Stronger compliance and audit readiness.
- Faster batch release and reduced risk of costly failures.
- With ProSolvr, organizations transform deviations into actionable insights, building a resilient, compliant, and efficient CAR T manufacturing ecosystem.
Use ProSolvr by smartQED to systematically mitigate challenges in the pharmaceutical industry and build long-term operational resilience.